Promising Least Invasive Heart Valve Replacement Operation
Several individuals ailing from a life-menacing cardiac problem called severe aortic stenosis can be benefited by a trial therapy which circumvents the need for open heart operation for replacing aortic valves, a novel research has indicated.
Nearly one and a half million people in the U.S. have aortic stenosis – an advancing constricting of the aortic valve which thwarts pumping of blood supply from the heart to the rest of the body & brains. Around 300000 of such individuals have an adequately acute problem that necessitates the valves to be artificially replaced. However open heart surgery could carry significant risk for around 1 in 3 patients, several of them being aged for whom there is presently no effectual therapy.
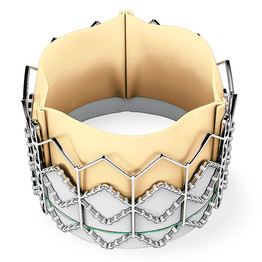 Edwards Lifesciences Corp, California has provided funding and is behind the development of this investigational treatment created for properly positioning a novel aortic valve sans major surgical intervention. The replaced valve is affixed to catheter and threading done via blood vessels till it is able to reach the heart.
Edwards Lifesciences Corp, California has provided funding and is behind the development of this investigational treatment created for properly positioning a novel aortic valve sans major surgical intervention. The replaced valve is affixed to catheter and threading done via blood vessels till it is able to reach the heart.
The trial appearing in the ‘New England Journal of Medicine’ did a follow-up of 358 aged patients who were around eighty-three years old. Placement of catheter conveyed valves were done for fifty percent of the entrants and the remaining were not given replacement valves. Rather management of their health condition was done by varied ways like widening their valve using a mini balloon.
Subsequent to a year, deaths occurred in 30.7 percent of the patients in whom replacement valves were placed which is way beneath the fifty percent fatality rate amongst patients among whom management of the heart problem was done sans placing novel valves.
The researchers arrived at a conclusion that valve placement via catheter delivery must be the novel benchmark of care among patients who are not candidates for undergoing open heart operation. Several of these researchers have links to Edwards Lifesciences via consultation charges and several ways.
However the trial additionally revealed an elevated incidence of strokes among patients who received the valves via catheter delivery. In just a month’s time following the method, five percent of such patients experienced major strokes in comparison to 1.1 percent of the study group among whom just management of their disorder was done. Moreover, there was a greater danger of vascular issues such as blood loss among patient sets who received replaced valves.
The co-main researcher of the trial, heart specialist Craig Smith from NY- Presbyterian Hospital pointed out that it was reasonable to state that the stroke incidence was more as compared to what all were anticipating and many folds more than deemed agreeable. However on the basis of the risk such aged and weak patients are facing when valve replacement is not done then it too is a satisfactory price to disburse.
Aortic valve replacement by Edwards Lifesciences is presently being employed among European patient groups where it vies with Medtronic promoted analogous device. The Minneapolis-based firm additionally garnered the United States regulatory acceptance in 2010 for a trans-catheter tool for replacing congenital flaw in the pulmonary valve – the foremost consent in the United States for a cardiac valve replacement sans open heart operation.
The device by Edwards of the United States trial called the Partner trial is prepared from tissues of cows and is seated within a metallic casing. Crimping of the device is done on a balloon catheter whose threading is done via an artery in the leg reaching the heart and then expanding it within the flawed valve.
Researchers state the high incidence of strokes amongst patients who received replacement valves can be because of several factors inclusive of weak health conditions of such patients. Many physicians state that mastering the method would take time & with passage of time a surgeon would enhance his/her skill.
Edwards aims to submit an application to the FDA in the concluding part of 2010 that can perhaps attain consent during the subsequent year.



